half life formula for first order reaction
Half Life Period Of First Order Reaction Click Here for Sample Questions Half-time or half life of a first order reaction is the time taken to reduce the concentration of the reactant to half of its initial value. For a first-order reaction the half.

Pin By Shanmukhi On Studies In 2022 Chemical Kinetics Chemistry Chemistry Class
Equations for Half Lives.

. Converting a half life to a rate constant. Answer 1 of 4. T ½ A o 2k For a first order reaction A products rate kA.
The half-life equations for a zeroth first and second order reaction can be derived from the corresponding integrated rate laws using the relationship given above. I found this statement. Therefore A t 1 2 A 0 at t 1 2.
Ln 2 0693 kt12. Rate k A 1. T 12 ln2k.
T ½ 1 k A o Top. The half-life of a second-order reaction is given by the formula 1kR 0. As the half life is always constant for a first order process then the rate constant of the reaction can be related to the half life by a simple equation.
Frac 1 A_02 frac 1 A_0 kt_ 12 frac 1 A_02 - frac 1 A_0 kt_ 12. How is that true. For a zero order reaction A products rate k.
The reaction order can be determined by measuring the half-life as a function of the concentrations of the reacting species. And for the half-life. Half Life of First Order Reactions First-Order Reactions We can derive an equation for determining the half-life of a first-order reaction from the alternate form of the integrated rate law as follows.
The half-life of a chemical reaction regardless of its order is simply the time needed for half of an initial concentration of a reactant to be consumed by the reaction. For a first order reaction t½ 0693 k and for a second order reaction t½ 1 k Ao. Notice that the half life does not depend on the reactant concentration.
Where k is the rate constant and ln2 is the natural log of 2 0693. The half-life of a first-order reaction is given as t 12 0693k. Determining a half life.
Now we have the following equation and can solve for eqt_ 12 eq. Half-life or t½ is the time that elapses before the concentration of a reactant is reduced to half its initial. Find more Chemistry widgets in WolframAlpha.
For the first order reaction you can plug the definition of the half life into the concentration-time reaction to obtain a neat relationship. What is the expression for Half-Life of a First Order ReactionHere I derive it from the integrated rate lawThe answer is t ln 2 kAsk me questions. For a first-order reaction.
For first order reaction we know that k 1t. The rate of a first-order reaction is proportional to the concentration of one reaction species A. AA_0 e-kt At half-life point A.
Determining reaction order using half-life. The half-life of a reaction is the time required for a reactant to reach one-half its initial concentration or pressure. The half-life of a reaction t 1 2 is the time required for an initial reactant concentration A 0 to decrease by one-half.
T ½ 0693 k For a second order reaction 2A products or A B products when A B rate kA 2. Ln 12A0A0 -kt12. For a zero order reaction the formula is t½ Ao 2k.
The half-life of a chemical reaction denoted by t 12 is the time taken for the initial concentration of the reactants to reach half of its original value. Now a first-order reaction is characterized by the fact that the rate of the reaction depends linearly on the concentration of one reactant. Rate constant is the coefficient of proportionality relating the rate of a chemical reaction at a given temperature to the concentration of reactant or product.
It is denoted by t12. Log e A 0 A At half life period t t12 and A A 0 2. Where The half-life of a reaction is referred to as t 12 unit - seconds The initial reactant concentration is referred to as R 0 in molL.
Therefore At t t 12 A A 0 2. Get the free Half Life Calculator first order reaction widget for your website blog Wordpress Blogger or iGoogle. You want to calculate the time it takes for the concentration to fall from A 0 to ½ A 0.
FraclnA_0A_tkt tfraclnA_0A_ttimesfrac1k. The First Order Half-Life calculator computes the first order half-life based on the temperature dependent rate constant. Substituting the values in the expression for the rate constant of half-life first-order reaction the.
The first-order reaction half-life equation is given by k 2303 t l o g R 0 R From the definition of the half-life of a first-order reaction at t t12 and R R 02. The formula for half-life in chemistry depends on the order of the reaction. Half-Life of a First-Order Reaction.
The rate law for this first order rate equation is where A is the concentration of radium-223 at time t and k is the rate constant 00606 day -1. Graphical relations and half lives. Half Life period of first order reaction is the time required for 50 percent completion of the reaction and is represented as t 05 ln 2 K or Half Life Period ln 2 Rate constant.

Pin By Marti Dietz On Workout Estimate Method Half Life

Chemical Kinetics And Half Life Online College Chemistry Courses Chemical Kinetics Study Chemistry Half Life

Electronic Configuration Of Actinides Actinium And The F Block Element Electron Configuration Configuration Element

Chemical Kinetics Half Life Definition Equations Chemical Kinetics Half Life Reaction Rate

Chemistry Crossword Puzzle Chemical Reactions Includes Answer Key Teaching Resources Chemical Reactions Teaching Chemistry Crossword

Freethinkers Overstanding Optics Waves Optics Is The Branch Of Physics Which Involves The Behaviour And Properties Of Physics Formulas Learn Physics Physics

Kinetics Ppt Chemical Kinetics Chemical Equation Enzyme Kinetics
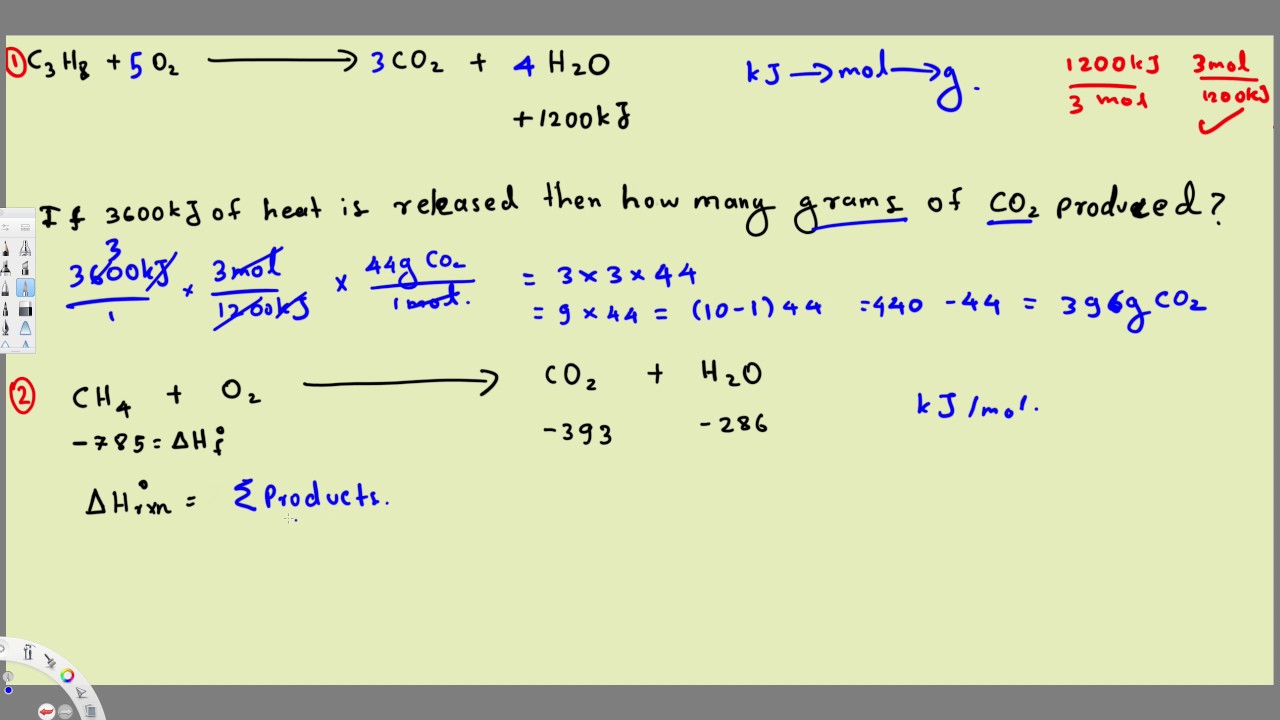
Thermochemistry Equations Formulas Practice Problems Example 2 Equations Practice Chemistry

Pin By Shanmukhi On Studies In 2022 Chemical Kinetics Chemistry Chemistry Class

Ncert Solutions For Class 12 Chemistry Chapter 4 Chemical Kinetics Cbse Tuts Chemicalkineticsclass12ncertsolutions Chemical Kinetics Chemistry Solutions

Half Lives Chemistry Lessons Teaching Chemistry Chemistry Experiments

Radioactive Isotopes Secondary Science College Chemistry Medical Library

In The Following Practice Exercise We Will Determine The Major Product Of A Grignard Reagent Wit Study Chemistry Organic Chemistry Organic Chemistry Reactions

Molecular Orbital Theory And Bond Order Molecular Electron Configuration Bond

12 Class Chapter 1 Solid State Chemistry Notes Notes Info Chemistry

Isotope Distribution Calculator And Mass Spec Plotter Teaching Chemistry Mass Spectrometry Ap Chem

Half Lives Chemistry Classroom Teaching Chemistry Chemistry Experiments

Zero Order Kinetics Chemistry Textbook Chemistry Classroom Chemistry Experiments
